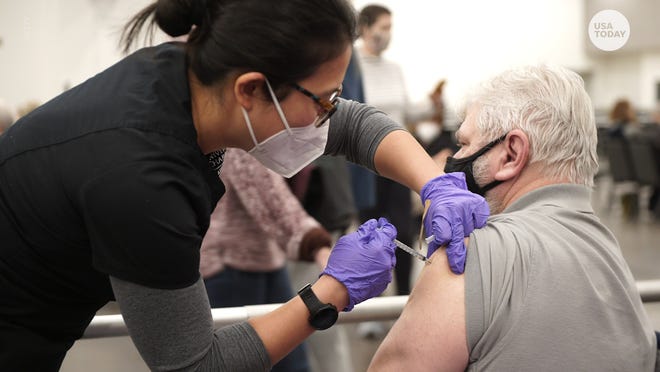
- A CDC advisory panel on Thursday recommended boosters for people 65 and older, long-term care residents and adults under 64 with medical conditions.
- People who have gotten two shots and decide not to get a third will still be considered fully vaccinated, the CDC said.
- The other two available COVID-19 vaccines in the U.S., from Moderna and Johnson & Johnson, have not yet been authorized for use as boosters.
The Centers for Disease Control and Prevention late Thursday endorsed booster shots for millions of older and high-risk Americans, opening a major new phase in the U.S vaccination drive against COVID-19.
CDC Director Dr. Rochelle Walensky signed off on a series of recommendations from a panel of advisers hours after the advisers said boosters for the Pfizer-BioNTech COVID-19 vaccine should be offered to people 65 and older, nursing home residents and those ages 50 to 64 who have risky underlying health problems. The extra dose would be given once they are at least six months past their last Pfizer shot.
But Walensky decided to make one recommendation that the panel had rejected.
The panel on Thursday voted against saying that people can get a booster if they are 18 to 64 years of age, are healthcare workers or have another job that puts them at increased risk of being exposed to the virus.
But Walensky disagreed and put that recommendation back in, noting that such a move aligns with an FDA booster authorization decision earlier this week.
People who have gotten two shots and decide not to get a third will still be considered fully vaccinated, the CDC said.
No one will need a doctor's note to walk into a pharmacy and request a third dose. They will just have to consider for themselves whether the benefit they will derive from a booster outweighs their personal risk. Several committee members said this will create problems with implementation and worried that it will add to the confusion many people feel about vaccines.
How does COVID-19 affect me?Don’t miss an update with the Coronavirus Watch newsletter.
The evidence is clear that most vaccinated Americans remain well protected by the shots they already received, committee members and CDC officials said.
More than 90% of those currently hospitalized with COVID-19 have not been vaccinated and the best way to combat the current pandemic is to give initial shots to those who have not had any, they said.
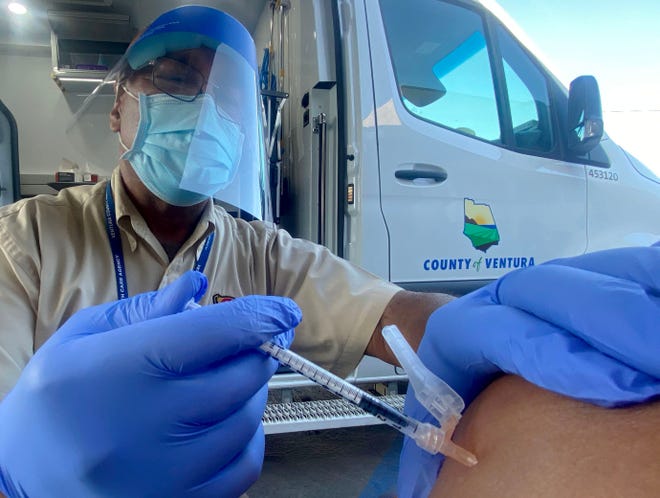
Lab research and data from Israel, where booster shots were made widely available this summer, suggests protection against COVID-19 infection begins to wane about six months after initial shots, though hospitalization for vaccinated people remains rare. Those over 65 are most at risk for severe disease, which is why the committee, called the Advisory Committee on Immunization Practices or ACIP, voted unanimously to recommend they receive boosters.
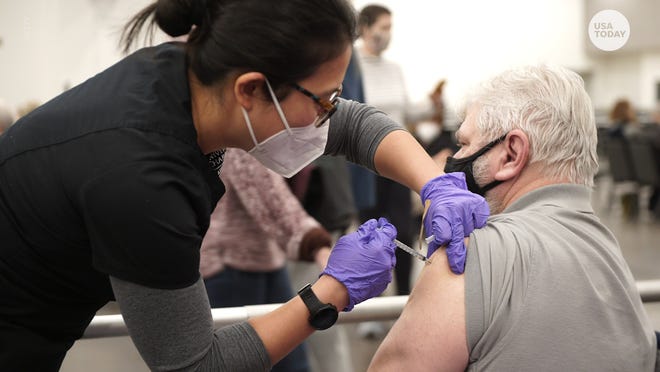
At the moment, only the vaccine made by Pfizer-BioNTech, which has been fully licensed under the name Comirnaty, will be available for boosters. There are plenty of available doses for whoever needs them, the Biden administration has said.
A booster from Pfizer-BioNTech would be the same dose at the same vaccine as the previous two doses.
Not enough research has been completed to say that it's safe to get initial doses of one vaccine and then switch to another, Dr. Doran Fink of the Food and Drug Administration told the committee Thursday.
The other two available vaccines have not yet been authorized for use as boosters.
Moderna has requested authorization for a lower dose of its initial vaccine to be used as a booster, which the FDA is currently considering and which could become available for boosters in coming weeks.
What we know:Who is considered 'high risk' and eligible for Pfizer-BioNTech's booster shots?
Tracking COVID-19 vaccine distribution by state:How many people have been vaccinated in the U.S.?
Johnson & Johnson, which has been a single-dose vaccine, released information this week showing that a second dose is safe and improves effectiveness, but it has not yet submitted a request to the government for authorization to provide boosters.
In a talk Thursday, National Institutes of Health director Dr. Francis Collins said he had gotten the Moderna vaccine and is waiting for results of an NIH trial testing whether it's safe to boost people with a different vaccine than they got the first time.
He expects results within the next month or so and in the meantime, people who have gotten two shots of Moderna or one of J&J.
“If you got the original immunizations, you’re in very good shape still," he said in a broadcast talk sponsored by Bloomberg Philanthropies.
"Even though we're a little worried about the deterioration and the effectiveness for people who got those doses back in January, they're still pretty darn good and a good reason not to panic or rush around and do something until we really have all the data and science.”
In its other decisions Thursday, the ACIP panel voted 13-2 in favor of booster doses at least six months after initial shots for people ages 50-64 who have underlying medical issues that put them at higher risk for severe COVID-19.
They were more divided on whether to support boosters for younger people, but decided 9-6 to recommend that people ages 18-49 with underlying medical issues get a booster shot at least six months after their initial doses.The committee did not specify the medical conditions, but generally those at higher risk for serious disease include people with obesity, diabetes and lung or heart disease.
Such a vague description of eligibility will make it very hard for state and local officials to plan for vaccinations, said Dr. William Moss, an epidemiologist and infectious disease pediatrician at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
"They won't know how many doses to order," said Moss, who is not a committee member.
Pfizer-BioNTech, Moderna and Johnson & Johnson:Comparing the COVID-19 vaccines
In their final vote, the committee decided not to support boosters for those whose jobs or living situations put them at higher risk of infection with COVID-19.
ACIP member Dr. Helen Keipp Talbot, an associate professor of medicine at Vanderbilt University in Nashville, Tennessee, had argued for vaccinating health care workers, because hospitals like hers, currently overwhelmed with COVID-19 cases, can't afford to have employees out sick.
"Giving the option for a third dose helps us maintain our staffing," she said.
She also said she favored a broad allowance for boosters, because "almost every American is at risk. We are either obese or have a medical problem or … we live with someone who's at high risk or we teach a group of kids that aren't eligible to get vaccined yet."
But others weren't comfortable offering vaccines to such a broad swath of the public.
"In my opinion, there's little marginal benefit to making this booster dose available at this time," said Dr. Beth Bell, a professor in the School of Public Health at the University of Washington in Seattle, who only supported booster doses for those 65 and older.
“Obviously the committee was worried this was a hole you could drive a truck through,” said Dr. Paul Offit, who directs the Vaccine Education Center at Children’s Hospital of Philadelphia and is not on the panel.
“It was just such a wide-open field that they just couldn’t go there,” added Norman Baylor, president and CEO, Biologics Consulting, and a member of the COVID-19 Vaccine Analysis Team, a group of vaccine experts who regularly make themselves available to the media.
Boosters do make a substantial difference, Collins said in a talk Thursday.
“The data looks really impressive that the boosters do, in fact, provide substantial reduction in infection, like a tenfold fold reduction just within 12 days after that booster," he said, "and also a reduction in the severe illness, which is the thing we're most concerned about and which was starting to appear in people who hadn't gotten their vaccination as recently as you might like.”
The chance of suffering a serious vaccine side effect differs by age and sex.
Younger people, particularly men, run a higher risk of one of the most serious side effects seen with these vaccines: swelling of the heart muscle, known as myocarditis.
Your questions, answered:When will the Pfizer-BioNTech COVID-19 vaccine be ready for kids?
According to the CDC, out of every million booster doses administered, 13 people –mostly men – ages 18-29 would be expected to develop myocarditis if the risk is the same as with second doses. In the oldest age group, booster doses would not be expected to cause any myocarditis.
There's no data yet available on whether boosters will limit transmission of the virus. People who have been infected after vaccination can still be contagious, but are far less likely to pass on the virus than those who aren't vaccinated, CDC data shows.
Health care providers tend to be younger and healthier than the general public, but are regularly exposed to COVID-19 in their communities and were eligible for their vaccines first, beginning last December, so their protection may have waned more, according to a CDC presentation.

Both healthy people and those with one or two underlying medical conditions remain well protected after their first two shots, according to CDC data.
But many vaccinated people are ready for a booster dose.
In five published surveys made public in August, 76% to 87% of vaccinated adults said they would get a booster dose if one were available. In one survey, this increased to 93% if a booster was recommended by their primary care provider.
People who are unvaccinated, though, said they would be more likely to reject vaccination if they knew that booster doses were available.
When will everyone be vaccinated for COVID-19? Here's how the vaccine rollout is going.
Although the committee recommended boosters at least six months after initial doses, that doesn't mean people need to run to get a third shot six months and one day after their first two, said Rachael Piltch-Loeb, a biostatistician at the Harvard T. H. Chan School of Public Health who is not on the committee.
“There is no magic cutoff whereby we see that today things look one way and tomorrow they look some more different,” she said. "Whether it's six months, seven months, eight months, you at the individual level may still have excellent protection, especially against severe infection.”
Near the end of 11 hours of meetings over two days, one of the committee members complained that the focus on boosters was misplaced. The real emphasis should be on getting people vaccinated for the first time, Talbot said.
"The hospitals are full because people aren't vaccinated," she said. "I feel like we're putting lipstick on frogs. This is not going to solve the pandemic."
Contributing: The Associated Press
Contact Karen Weintraub at [email protected] and Elizabeth Weise at [email protected].
Health and patient safety coverage at USA TODAY is made possible in part by a grant from the Masimo Foundation for Ethics, Innovation and Competition in Healthcare. The Masimo Foundation does not provide editorial input.
Source link










