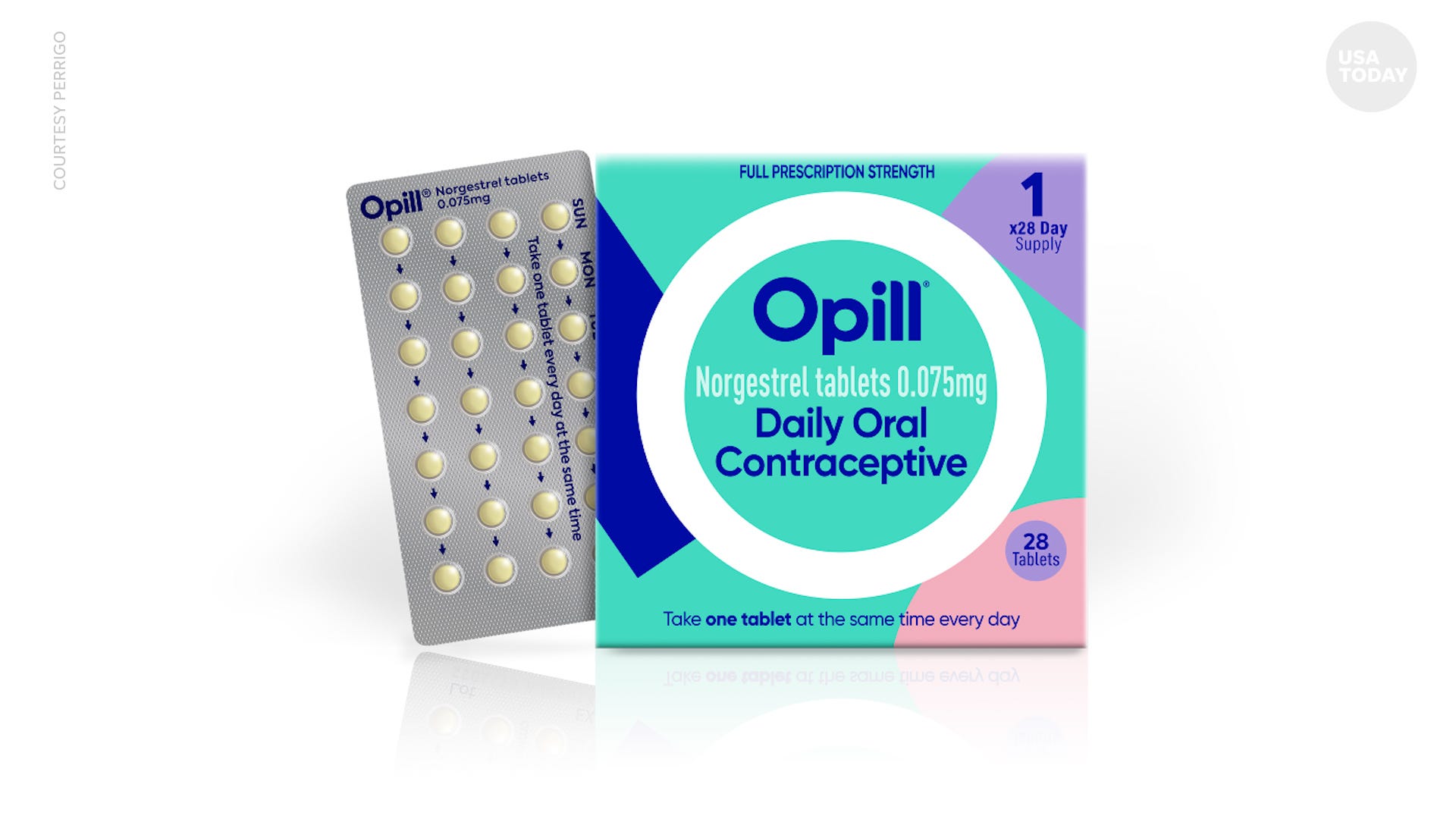

WASHINGTON – Federal officials have approved the nation's first over-the-counter birth control pill, making it the first daily oral contraceptive to be made available in the U.S. without a prescription in a move long called for by reproductive rights organizations and leading medical associations.
The Food and Drug Administration on Thursday signed off on Opill (norgestrel), a once-a-day tablet available by prescription since 1973 that will soon be readily available to consumers online and at drug stores, grocery stores and convenience stores.
“Today’s approval marks the first time a nonprescription daily oral contraceptive will be an available option for millions of people in the United States,” said Patrizia Cavazzoni, director of the FDA’s Center for Drug Evaluation and Research.
HRA Pharma, a French drugmaker owned by Ireland pharmaceutical company Perrigo, applied for over-the-counter status last summer.
"For a product that has been available for the last 50 years, that has been used safely by millions of women, we thought it was time to make it more available," said Frederique Welgryn, HRA’s chief strategy officer, said at the time.
Opill should hit shelves early next year
Hormone-based pills are the most common form of birth control in the U.S., used by tens of millions of women since the 1960s. Before Thursday's announcement, all had required a prescription.
Opill is expected to be more effective than other available nonprescription methods in preventing unintended pregnancies, Cavazzoni said.
Perrigo executives said the company will spend the rest of the year manufacturing the pill and its packaging so it can be available in stores nationwide and online by early next year. There will be no age restrictions on sales.
Opill’s over-the-counter status could go a long way toward removing barriers to access since people will be able to get an oral contraceptive without the need to first see a health care provider. Teens and girls, women of color and those with low incomes report greater hurdles in getting prescriptions and picking them up.
The over-the-counter contraceptive could also ultimately lessen the number of unintended pregnancies and their potential negative impacts.
Nearly half of the nation’s 6.1 million pregnancies each year are unintended, the FDA said. Such pregnancies are linked to negative maternal and perinatal outcomes – making it less likely, for instance, that a woman will get early prenatal care or raise the risks of preterm deliveries.
Nonprescription contraceptives are increasingly available
Nonprescription contraceptive pills are available around the world, including in South America, Asia and Africa. Last year, a nonprescription birth control pill was approved for the first time in the United Kingdom.
Opill uses the synthetic hormone progestin to block sperm from the cervix, preventing pregnancy. Most other birth control pills use progestin and estrogen, and progestin-only pills like Opill are often recommended for people who can't take combination pills because of health reasons.
Norgestrel’s efficacy was established through its original approval for prescription use in 1973. The FDA required HRA Pharma to demonstrate that the drug’s safe and effective use as a nonprescription drug, and studies showed that “a high proportion of consumers understood the label instructions, supporting their ability to properly use the drug,” the FDA said. “When properly used, Opill is safe and effective.”
The FDA has faced pressure from Democratic politicians, health advocates and medical professionals to ease access to birth control. The American Medical Association and the leading professional society for obstetricians and gynecologists backed Opill's application for over-the-counter status.
Contributing: Christine Fernando and The Associated Press
Source link