A New York-based company has just filed to patent the world’s first vaccine that would protect against both COVID and the seasonal flu, and it would be administered as a nasal spray.
The vaccine, called deltaFLU, would differ from the current mRNA vaccine in that it would contain a live COVID spike protein, so rather than instructing your body to produce an immune response as mRNA does, it would use its normal process of creating antibodies.
“We’ve gotten it to the point where we can manufacture it at a large scale and get it going really rapidly,” said Amy Aspelund, vice president of research and development for Vivaldi Biosciences, the company producing the combination vaccine.
Researchers estimate that COVID-19 has around 30,000 nucleotides, the building blocks of DNA. The deltaFLU vaccine, however, would only contain around 200-300 of those nucleotides, so the sample would be small enough to allow your body to recognize the virus without being overwhelmed by it.
The COVID-19 cultures would also be without NS1, the key protein that allows viruses to circumvent our body’s natural immune response. It would mean the body would be able to produce antibodies for the virus, without it replicating within the body.
“I’ve been working for bioscience companies for the last 20 years, so it is surreal,” said Aspelund. “In the first SARS pandemic we had a grant funded to do this exact thing that we’re doing now, but by the time we got around to doing it the pandemic was over and the project got abandoned, so we just kind of picked it up and ran with it.”
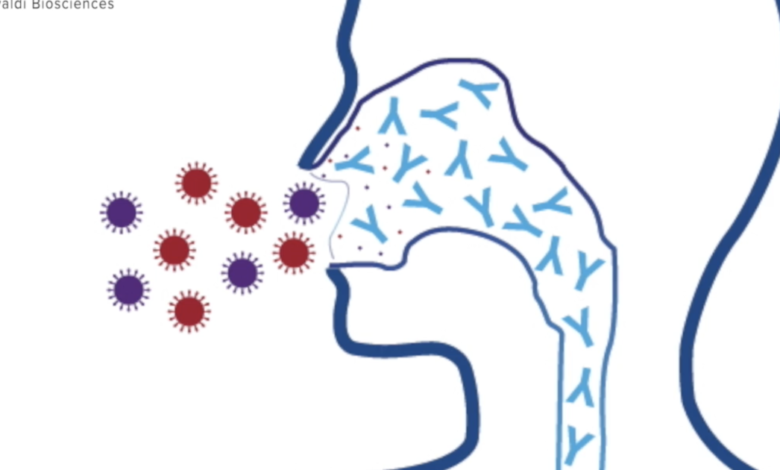
Aspelund says the fact that this vaccine would be administered as a nasal spray is yet another defense mechanism against COVID-19 and the seasonal flu. The idea is that by introducing this vaccine through the nose, your body would create antibodies at COVID’s main entry point, essentially creating a blockade so the virus could not get in. In theory, it would mean no breakthrough infections for vaccinated individuals.
Vivaldi Biosciences says it has had four successful trials of its deltaFLU vaccine within animals that prove its effectiveness and safety.
Once the patent application is approved, Aspelund says it would need to go through human trials before it could be produced and used commercially.






