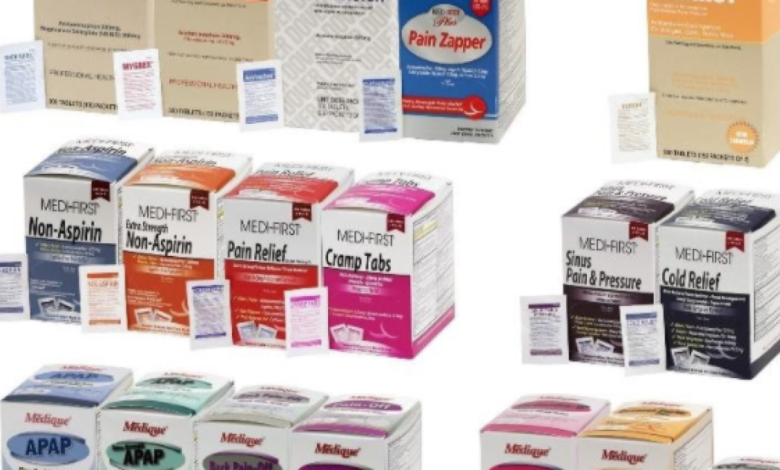
An over-the-counter drug sold exclusively on Amazon.com is being recalled because it failed to meet child-resistant packaging requirements.
The United States Consumer Product Safety Commission said Medique recalled the 31 products because the packaging is not child-resistant, which poses a risk of poisoning if young children swallow the contents.
Medique said over 143,000 drugs that are being recalled were purchased on or after June 1, 2018.
The products being recalled are listed in the table below:
| Product | Drug | Package Type | # of Packets |
| Medi-First Non-Aspirin Acetaminophen | acetaminophen (325 mg) | 2 tablets packet |
50 250 |
| Medi-First Extra Strength Non-Aspirin Acetaminophen | acetaminophen (500 mg) | 2 tablets packet |
50 125 250 |
| Medi-First Sinus Pain & Pressure | acetaminophen (500 mg) | 2 tablets packet |
50 125 250 |
| Medique APAP | acetaminophen (325 mg) | 2 tablets packet | 250 |
| Medique Extra Strength APAP | acetaminophen (500 mg) | 2 tablets packet |
50 125 250 |
| Medique Back Pain-Off | acetaminophen (250 mg) | 2 tablets packet |
50 100 250 |
| Medique CCP Caffeine Fee | acetaminophen (325 mg) | 2 tablets packet |
50 250 |
| Medi-First Cold Relief | acetaminophen (325 mg) | 2 tablets packet |
50 125 250 |
| Medique Cramp Tabs | acetaminophen (325 mg) | 2 tablets packet |
50 125 250 |
| Medique Decorel Forte Plus | acetaminophen (325 mg) | 2 tablets packet |
50 250 |
| Medique Medicidin-D | acetaminophen (325 mg) | 2 tablets packet |
50 100 250 |
| Dover Aminofen | acetaminophen (325 mg) | 2 tablets packet | 250 |
| Otis Clapp Back Quell | acetaminophen (200 mg) | 2 tablets packet | 150 |
| Otis Clapp Mygrex | acetaminophen (500 mg) | 2 tablets packet | 150 |
| Otis Clapp Valihist | acetaminophen (325 mg) | 2 tablets packet | 150 |
| Medi-First Pain Relief Extra Strength | acetaminophen (110 mg)aspirin (162 mg) | 2 tablets packet |
50 100 250 |
| Medi-First Plus Pain Zappers | acetaminophen (250 mg)aspirin (250 mg) | 2 tablets packet |
50 125 |
| Medique Pain-Off | acetaminophen (250 mg)aspirin (250 mg) | 2 tablets packet |
50 100 250 |
| Medi-First Aspirin | aspirin (325 mg) | 2 tablets packet |
50 125 250 |
| Medi-First Plus Aspirin | aspirin (325 mg) | 2 tablets packet |
50 125 |
| Medique Aspirin | aspirin (325 mg) | 2 tablets packet |
12 100 250 |
| Medique Diphen | diphenhydramine (25 mg) | 1 tablet packet |
24 200 |
| Medi-First Ibuprofen | ibuprofen (200 mg) | 2 tablets packet |
4 50 125 250 |
| Medique I-Prin | ibuprofen (200 mg) | 2 tablets packet |
3 100 250 |
| Dover Addaprin | ibuprofen (200 mg) | 2 tablets packet | 250 |
| Medi-First Burn Cream with Lidocaine | lidocaine (0.9 grams) | packets | 25 |
| Medi-First Burn Spray | lidocaine HCl (2%) | 2 oz bottle | -- |
| Medi-First Blood Clotting Spray | lidocaine (4%) | 3 oz bottle | -- |
| Ecolab Burn Cream | lidocaine (0.9 grams) | packets | 25 |
| Medique Diamode | loperamide HCl (2 mg) | 1 tablet packet |
6 50 100 |
| Medique Mediproxen | naproxen sodium (220 mg) | 1 tablet packet |
50 100 |
The expiration date for tablets and creams can be found on either the container carton's top or side panels in the format.
For products in spray bottles, the same format's expiration date is located on the bottle's front.
The expiration date is found on the bottom for the spray cans.
To receive a refund, consumers should contact Medique for information on how to dispose of the product and receive a full refund.
No incidents or injuries have been reported.







